Service Hotline: 400-688-8218
Product show
Product & Marketing
Product & Marketing
Hangzhou Yuanda Biopharmaceutical Co., Ltd. adheres to the people-oriented purpose, adheres to the management concept of "production quality first, marketing integrity and pragmatism", and is committed to continuous development and innovation, and has successively won the honorary titles of "Advanced Enterprise for Investment in Hangzhou", "Advanced Enterprise of Harmonious Labor Relations in Zhejiang Province", "AAA Credit Enterprise", "The Most Growing Small and Medium-sized Industrial Enterprise in Hangzhou", etc., and strives for the people of the whole country to use cutting-edge biological products with high quality and high price!
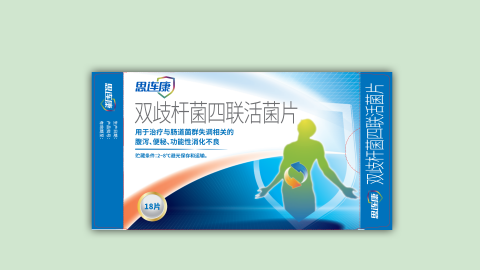
The following is the revised version of the latest product manual of Siliankang (Bifidobacterium quadruple viable tablets) produced by Hangzhou Yuanda Biopharmaceutical Co., Ltd. on December 16, 2022;
Picture Version:
Transcript:
Date of approval: 08 December 2008
Last revision date: (1) 2009-03-05; (2) 2011-01-19; (3) 2015-03-12; (4) 2015-07-06; (5) 2015-08-04; (6) October 11, 2017; (7) 2018-07-27; (8) 2018-08-08; (9) November 20, 2018; (10) 2019-04-15; (11) 2020-06-02; (12) 2020-07-13; (13) 2020-08-24; (14) 2021-06-15; (15) 2022-09-15; (16) December 16, 2022
Silicon
Bifidobacterium quadruple viable tablets instructions
Please read the instructions carefully and use under the guidance of a physician
If the packaging leaks and the properties of the drug change, do not take it, and contact the manufacturer in time.
If the outer packaging box is scratched, please do not buy.
【Drug Name】
Generic name: Bifidobacterium quadruple viable tablets
Product Name: Silicon
Product Name:Combined Bifidobacterium ,Lactobacillus,Enterococcus and Bacillus cereus Tablets,Live
Hanyu Pinyin: Shuangqiganjun Silian Huojun Pian
【Ingredients】This product is a compound preparation, the main components are: Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, Bacillus cereus.
【Properties】This product is a white-like tablet.
【Indications】This product is used for the treatment of diarrhea, constipation, and functional dyspepsia related to intestinal flora disorders.
[Specification] Each piece weighs 0.5 grams. Among them, Bifidobacterium infantis, Lactobacillus acidophilus and Enterococcus faecalis should not be less than 0.5×10^6CFU, respectively; Bacillus cereus should not be less than 0.5×10^5 CFU.
【Dosage】Oral, 3 times a day, 3 tablets at a time, severe cases can be doubled or according to the doctor's advice. Take after meal with warm water or warm milk.
【Adverse reactions】No obvious adverse reactions were found in the pre-marketing clinical trials of this product. The following adverse reactions were reported after marketing: rash, itching, urticaria, night sweats, fatigue, drowsiness, bloating, abdominal pain, vomiting, loose stools, diarrhea. Given that these reactions are spontaneously reported by an indeterminate number of users, it is not possible to accurately assess their frequency or determine causal relationship with drug exposure.
Contraindications are not clear.
【Precautions】
1. This product should not be stored for a long time after opening the bag, and should be taken as soon as possible.
2. It is forbidden when the properties of this product change.
3. Children must be under adult supervision.
[Medication for pregnant and lactating women] is not clear.
【Children's Medicine】
1. Children (including newborns, infants and young children) should follow the doctor's advice.
2. In order to prevent dysphagia, the tablets can be crushed or chewed.
3. Because this product is a live bacterial preparation, the temperature should not be higher than 40 °C when taken with warm boiled water or warm milk.
【Geriatric Drugs】The literature on post-marketing clinical application does not suggest special safety issues. For geriatric medications, please follow your doctor's advice.
【Drug Interactions】
1. Chloramphenicol, cephalosporin, erythromycin and penicillin have inhibitory effect on the live bacteria in this product.
2. Bismuth, tannic acid, medicinal carbon, tincture, etc. can inhibit, adsorb or kill inactivated bacteria, and should not be combined.
[Drug overdose] is not known.
【Pharmacology and Toxicology】Bifidobacterium infantis, Lactobacillus acidophilus and Enterococcus faecalis in this product are the normal intestinal flora of healthy human beings, and direct supplementation can inhibit some pathogenic bacteria in the intestine, maintain normal intestinal peristalsis, and adjust the balance of intestinal flora. Bacillus cereus colonizes the intestine and consumes oxygen, creating an anaerobic environment for anaerobic bacteria such as Bifidobacteria, and promoting the growth and reproduction of anaerobic bacteria such as Bifidobacteria.
【Pharmacokinetics】After this product is orally taken into the intestine, it will grow, multiply and colonize in the intestine. Among them, Bacillus cereus is not a member of the normal human intestinal flora, and is excreted with feces after colonization in the intestine for 48 hours. The other three bacteria are all normal flora in the human intestine, and they are generally colonized for more than 10 days to achieve balance.
【Storage】Store and transport at 2~8°C in the dark.
【Packing】PVC, aluminum foil; 9 pcs× 2 plates/box, 9 pcs× 4 plates/box, 9 pcs× 6 plates/box, 12 pcs× 2 plates/box, 12 pcs× 4 plates/box, 15 pcs× 1 plate/box, 15 pcs×2 plates/box, 15 pcs× 4 plates/box, 15 pcs×6 plates/box, 15 pcs× 12 plates/box, 15 pcs×12 plates/box.
[Expiration date] 24 months.
【Executive standard】WS4-(S-001)-2003Z (4 of which is subscript)
【Approval number】National medicine quasi-word S20060010
【Marketing Authorization Holders】
Marketing authorization holder: Hangzhou Yuanda Biopharmaceutical Co., Ltd
Drug Marketing Authorization Holder Address: No. 63, Jiuhuan Road, Shangcheng District, Hangzhou City, Zhejiang Province (Building 14 of the Standard Workshop)
Postal code: 310019
Phone: 0571-28021692
Fax: 0571-28020121
National service telephone: 400-6888-218
【Manufacturer】
Company name: Hangzhou Yuanda Biopharmaceutical Co., Ltd
Address: No. 63, Jiuhuan Road, Shangcheng District, Hangzhou City, Zhejiang Province (Building 14 of the standard workshop)
Postal code: 310019
Phone: 0571-28021692
Fax: 0571-28020121
National service telephone: 400-6888-218
扫二维码用手机看
Product & Marketing
New


Product & Marketing
Official QR Code


Contact Us
Copyright ©Hangzhou Yuanda Biopharmaceuticals Co., Ltd. 浙ICP备09055220号-1 互联网药品信息服务资格证书(浙)-非经营性-2020-0037 Xinnet technical support